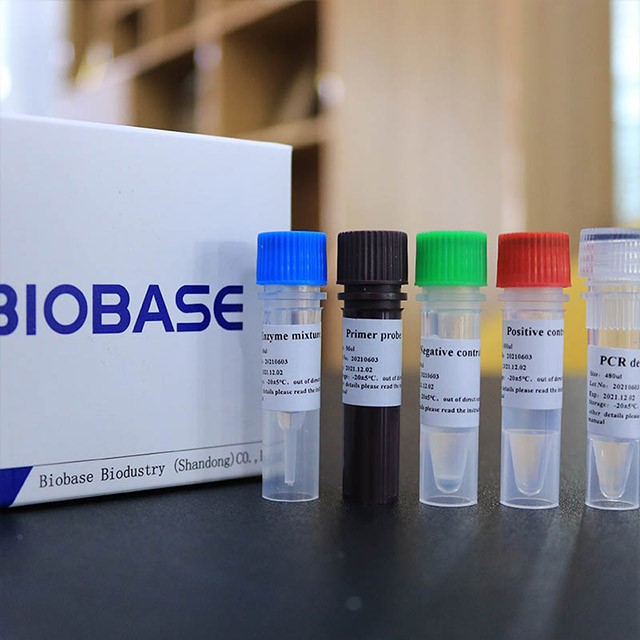
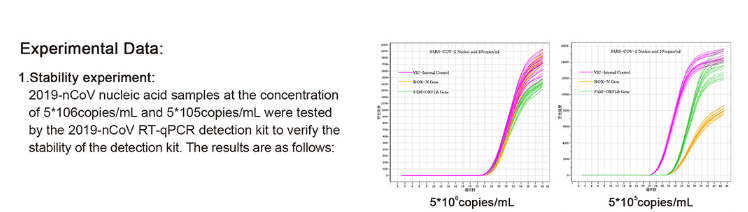
2019-nCoV RT-qPCR Detection Kit
₱ 100.00
Brand: Biobase -CHINA
Introduction: This product selects the ORF1ab and N gene regions of 2019-novel coronavirus, and designs two sets of specific primers and fluorescent probes that cover two sites of the gene at the same time. In order to control the entire extraction and detection process, human β-Globin gene was act as a non-competitive internal control during the extraction and detection process. The RT-PCR reaction solution is pre-mixed, which is convenient, efficient and easy to operate. It utilizes high-efficiency Taq DNA Polymerase and has strong stability. The kit could be used for auxiliary diagnosis and epidemiological surveillance of 2019-novel coronavirus infection. Application: Medical and health industry, food industry, biological industry, scientific research, etc.
NOTE: No Exchange No Return Learn More
Warranty: Not applicable, except in cases of incorrect results from the chart. In such instances, we will replace the product upon receipt of proof in the form of videos and photos.
Quality comes first and our products deliver accurate results.